The Philosophical Considerations of Patient Enrollment in Cancer Clinical Trials
- M.Richman M.D.

- 5 days ago
- 3 min read
Cancer clinical trials offer patients an avenue for cutting-edge medical treatments and a chance to contribute to advancing scientific knowledge. Yet, the decision to become a participant in a clinical trial is complex and nuanced, requiring careful consideration of several factors, including the disease stage, treatment history, and nature of the trial itself. Today’s article seeks to explore the types of patients who might consider enrolling in a clinical trial, particularly in the context of cancer care, and the philosophical implications of such a decision.
The Newly Diagnosed Patient
For a patient who has just been diagnosed with cancer, enrolling in a clinical trial may appear as both an opportunity and a daunting prospect. On one hand, novel therapies can offer hope where conventional treatment might not suffice. These patients often experience high levels of emotional distress, characterized by uncertainty about the future and anxiety over their prognosis. A clinical trial, especially those related to new drug treatment such a a novel Antibody-drug Conjugate (ADC) or a pioneering approach like Histotripsy for a malignant liver tumor, can provide a sense of hope and opportunity to be at the forefront of medical innovation.
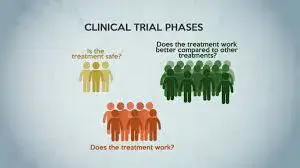
However, there are significant philosophical and ethical considerations at play. The exploratory nature of early-phase trials, such as Phase I, primarily assesses safety rather than efficacy, which may not align with the urgent need for effective treatment in newly diagnosed patients. The philosophical question arises: Is it justifiable for a patient in need of immediate care to participate in a trial that may not provide the benefit they are seeking? The choice of a newly diagnosed patient to enroll can be viewed through the lens of existential questions of hope, agency, and the quest for transformative experiences in moments of crisis.
The Patient with No Available Treatments
Conversely, patients who have exhausted all conventional treatment options often face a different existential dilemma. They may be grappling with a terminal diagnosis or have experienced disease progression after multiple therapies. For them, enrolling in a clinical trial may represent their last opportunity for intervention. Their situation can evoke poignant philosophical themes surrounding the concepts of dignity, desperation, and the autonomy of making profoundly important personal choices.
In this context, Phase II and Phase III clinical trials become relevant considerations. Phase II trials typically assess effectiveness and further evaluate safety in a larger group, ideally offering the prospect of better outcomes. Phase III trials, which compare a new treatment with the standard of care, often provide a more balanced view of potential benefits versus risks. Yet, for patients at the end of traditional treatment options, the distinction between phases may lose relevance; the mere act of participating may offer emotional solace and a semblance of control amidst the uncertainty of terminal illness.
The Rare Cancer Patient
Rare cancer patients face a particularly unique set of challenges. Due to limited treatment options and lack of extensive research, these patients may find that traditional routes are insufficient. For them, clinical trials may offer the only viable pathway toward receiving innovative treatments specifically targeting their type of cancer. The philosophical implications are particularly rich here, reflecting on the nature of medical advancement and societal obligations toward those suffering under the burden of necessity.
Participating in clinical trials designed for rare cancers can also evoke feelings of being a pioneer or a contributor to medical knowledge. The rich philosophical themes of altruism, shared humanity, and the quest for knowledge underscore their decision to enroll, resulting in a question of whether one’s willingness to take risks can benefit both the individual and society at large.

In summary, the decision for any patient, whether newly diagnosed, at the end of conventional options, or facing a rare cancer to enroll in a clinical trial is marked by an intricate web of ethical and philosophical challenges. The patient must weigh the promise of hope against the uncertainties inherent in experimental treatment, all while considering their own desires for dignity, agency, and meaning. Ultimately, the question of which type of patient should consider enrolling in a clinical trial cannot be answered universally. Each situation presents its own unique challenges and potential rewards. What emerges is a nuanced portrait of patient participation informed by individual circumstances, ethical considerations, and the overarching human quest for knowledge and healing in the face of life's most formidable adversities.





Comments